
Adapted from Rosmarin et al. 2022.
Intended for licensed healthcare professionals located in the United Kingdom and Republic of Ireland only
Opzelura® is indicated for the treatment of non-segmental vitiligo
with facial involvement in adults and adolescents from 12 years of age1

Opzelura® (ruxolitinib) was studied in two identical double-blind, vehicle-controlled, randomised, Phase III clinical trials, TRuE-V1 and TRuE-V2 (Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2). 674 adult and adolescent patients from 12 years of age with non-segmental vitiligo with facial involvement were enrolled in the two trials which investigated ruxolitinib, a Janus kinase (JAK) inhibitor for the treatment of non-segmental vitiligo.2

Adapted from Rosmarin et al. 2022.
Scroll right if necessary

TRuE-V1 and TRuE-V2 were multinational, randomised Phase III clinical trials assessing ruxolitinib in vitiligo, which took place in over 101 centres in countries including the United States, Canada and Europe (Bulgaria, France, Germany, Italy, Netherlands, Poland and Spain). 2
In TRuE-V1 and TRuE-V2, patients were randomised in a 2:1 ratio to Opzelura® treatment or vehicle cream. 450 patients were assigned to Opzelura® treatment, whilst 224 were assigned the vehicle cream, twice daily (BID) for 24 weeks. After Week 24, there was a 28-week open-label extension, where patients originally assigned to the vehicle could switch to Opzelura®.2
Key eligibility criteria for TRuE-V1 and TRuE-V2 participants included:2
Key exclusion criteria for TRuE-V1 and TRuE-V2 participants included:2
The primary and secondary endpoints in the TRuE-V1 and TRUE-V2 clinical trials used the VASI scoring index to assess improvement in depigmented skin in participants with non-segmental vitiligo.
Other prespecified secondary endpoints include rates of treatment-related adverse events (TRAEs)
The VASI score is calculated by (surface area of vitiligo lesions in hand units) X (degree of depigmentation of lesions).3,4
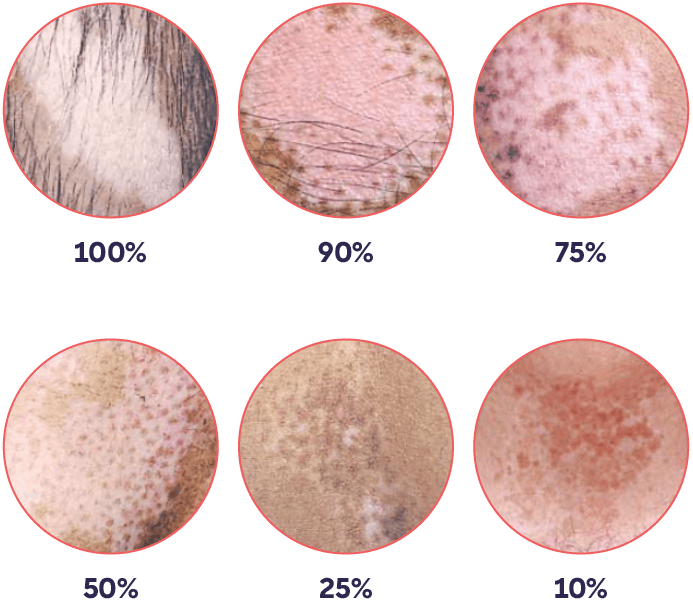
Participants were required to have:
The TRuE-V1 and TRuE-V2 trials included all Fitzpatrick skin types, with 89% of participants having types II–IV. The mean (±SD) age of patients in the safety populations was 40.2±15.9 years in TRuE-V1 and 38.9±14.3 years in TRuE-V2.2
A total of 10.9% of the patients in TRuE-V1 and 10.5% of those in TRuE-V2 were 12–17 years of age, and 54.5% and 57.1% respectively, of the patients were 40 years of age or younger; 56.4% and 50.1% respectively, of the patients were girls or women.2

Adapted from Rosmarin et al. 2022.
Scroll right if necessary

|
Vehicle cream (n=109) |
Opzelura (n=221) | |
|---|---|---|
| Race or ethnic group, n (%)‡ | ||
| White | 96 (88.1) | 180 (81.4) |
| Black | 4 (3.7) | 11 (5.0) |
| Asian | 4 (3.7) | 5 (2.3) |
| Other | 2 (1.8) | 9 (4.1) |
| Not reported | 3 (2.8) | 16 (7.2) |
| Geographic region, n (%) | ||
| North America | 73 (67.0) | 147 (66.5) |
| Europe | 36 (33.0) | 74 (33.5) |
| F-VASI, mean±SD§ | 1.0±0.6 | 0.9±0.6 |
| T-VASI, mean±SD¶ | 6.4±1.9 | 6.5±2.0 |
| Facial BSA affected by vitiligo, %** | 1.2±0.7 | 1.1±0.7 |
| Total BSA affected by vitiligo, % | 7.2±2.0 | 7.3±2.0 |
| Received diagnosis in childhood, n (%) | 34 (31.2) | 72 (32.6) |
| Other autoimmune disorders, n (%)†† | 18 (16.5) | 53 (24.0) |
| Thyroid disorders | 17 (15.6) | 50 (22.6) |
| Juvenile diabetes mellitus | 1 (0.9) | 0 |
| Pernicious anaemia | 0 | 1 (0.5) |
| Other | 1 (0.9) | 5 (2.3) |
| Previous therapy, n (%)‡‡ | 61 (56.0) | 131 (59.3) |
| Topical calcineurin inhibitors | 31 (28.4) | 72 (32.6) |
| Topical glucocorticoids | 28 (25.7) | 67 (30.3) |
| NB-UVB phototherapy | 20 (18.3) | 41 (18.6) |
| Excimer laser therapy | 8 (7.3) | 18 (8.1) |
| PUVA photochemotherapy | 4 (3.7) | 8 (3.6) |
| Vitamin D derivatives | 2 (1.8) | 4 (1.8) |
| Other | 11 (10.1) | 24 (10.9) |
Adapted from Rosmarin et al. 2022.
Scroll right if necessary

| Baseline characteristics for TRuE-V2 | ||
|---|---|---|
|
Vehicle cream (n=115) |
Opzelura (n=228) | |
| Age (years) mean±SD | 39.8±12.1 | 38.4±15.2 |
| Age group, n (%) | ||
| 12–17 | 6 (5.2) | 30 (13.2) |
| 18–64 | 106 (92.2) | 186 (81.6) |
| ≥65 | 3 (2.6) | 12 (5.3) |
| Female sex, n (%) | 60 (52.2) | 112 (49.1) |
| Race or ethnic group, n (%)‡ | ||
| White | 93 (80.9) | 182 (79.8) |
| Black | 5 (4.3) | 12 (5.3) |
| Asian | 7 (6.1) | 12 (5.3) |
| Other | 7 (6.1) | 19 (8.3) |
| Not reported | 3 (2.6) | 3 (1.3) |
| Fitzpatrick skin type, n (%)§§ | ||
| I | 1 (0.9) | 2 (0.9) |
| II | 32 (27.8) | 57 (25.0) |
| III | 45 (39.1) | 89 (39.0) |
| IV | 25 (21.7) | 55 (24.1) |
| V | 10 (8.7) | 17 (7.5) |
| VI | 2 (1.7) | 8 (3.5) |
| Geographic region, n (%) | ||
| North America | 83 (72.2) | 160 (70.2) |
| Europe | 32 (27.8) | 68 (29.8) |
| F-VASI, mean±SD§ | 0.8±0.5 | 0.9±0.5 |
| T-VASI, mean±SD¶ | 7.0±2.2 | 6.8±2.1 |
| Facial BSA affected by vitiligo, %** | 0.9±0.6 | 1.0±0.6 |
| Total BSA affected by vitiligo, % | 7.7±2.0 | 7.4±2.0 |
| Duration of disease (years) | 16.0±11.6 | 15.9±12.1 |
| Received diagnosis in childhood, n (%) | 43 (37.4) | 96 (42.1) |
| Disease stability, n (%)† | ||
| Stable | 88 (76.5) | 166 (72.8) |
| Progressive | 27 (23.5) | 62 (27.2) |
| Other autoimmune disorders, n (%)†† | 18 (15.7) | 37 (16.2) |
| Thyroid disorders | 15 (13.0) | 35 (15.4) |
| Juvenile diabetes mellitus | 0 | 0 |
| Pernicious anaemia | 0 | 0 |
| Other | 6 (5.2) | 5 (2.2) |
| Previous therapy, n (%)‡‡ | 76 (66.1) | 143 (62.7) |
| Topical calcineurin inhibitors | 37 (32.2) | 74 (32.5) |
| Topical glucocorticoids | 28 (24.3) | 66 (28.9) |
| NB-UVB phototherapy | 27 (23.5) | 52 (22.8) |
| Excimer laser therapy | 14 (12.2) | 16 (7.0) |
| PUVA photochemotherapy | 8 (7.0) | 15 (6.6) |
| Vitamin D derivatives | 1 (0.9) | 0 |
| Other | 14 (12.2) | 34 (14.9) |
Adapted from Rosmarin et al. 2022.
*A rating of “a lot less noticeable” or “no longer noticeable”.
†Determination of disease stability was based on investigator judgement.
‡Race and ethnic group were reported by the patient. “Other” includes American Indian or Alaska Native, Native Hawaiian or Pacific Islander and other.
§Scores on the F-VASI range from 0–3, with higher scores indicating a greater area of facial depigmentation.
¶Scores on the T-VASI range from 0–100, with higher scores indicating a greater area of total body depigmentation.
**Shown is the percentage of total BSA.
††Patients could report multiple autoimmune disorders.
‡‡Patients could have used multiple previous lines of therapy. “Other” includes other types of phototherapy, oral glucocorticoids, surgical techniques and other.
§§Fitzpatrick skin types range from I to VI: type I indicates pale white; type II, white; type III, light brown; type IV, moderate brown; type V, dark brown; and type VI, deeply pigmented brown to black.
BID, twice daily; BSA, body surface area; F-BSA, facial body surface area; F-VASI, Facial Vitiligo Area Scoring Index; F-VASI50, ≥50% reduction from baseline in Facial Vitiligo Area Scoring Index; F-VASI75, ≥75% reduction from baseline in Facial Vitiligo Area Scoring Index; F-VASI90, ≥90% reduction from baseline in Facial Vitiligo Area Scoring Index; JAK, Janus kinase; NB-UVB, narrowband ultraviolet B; PUVA, psoralen ultraviolet A; SD, standard deviation; T-BSA, total body surface area; TCI, topical calcineurin inhibitor; TCS, topical corticosteroid; TRAE, treatment-related adverse event; TRuE-V, topical ruxolitinib evaluation in vitiligo study; T-VASI, Total Vitiligo Area Scoring Index; T-VASI50, ≥50% reduction from baseline in Total Vitiligo Area Scoring Index; VASI, Vitiligo Area Scoring Index; VNS, Vitiligo Noticeability Scale.
UNITED KINGDOM
Adverse events should be reported.
Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
Adverse events should also be reported to Incyte by calling 00-800-0002-7423.
REPUBLIC OF IRELAND
Adverse events should be reported.
Reporting forms and information can be found at HPRA Pharmacovigilance:
www.hpra.ie. Adverse events should also be reported to Incyte by calling
1800‑456‑748.
This is a promotional website intended for licensed healthcare
professionals in the United Kingdom or Republic of Ireland.
You are now leaving the Incyte OPZELURA® website. This link will take you to a site that is not owned or maintained by Incyte. Incyte is not responsible for the information contained on third–party sites. Would you like to continue?
You are leaving the site https://www.opzelura.co.uk/. This link will take you to the site https://www.incyte.com, owned by Incyte Corporation and managed by the company.