Opzelura® – redefining the future


Can I talk to my doctor about the first approved treatment option for vitiligo?

Now you can.*
Opzelura® topical cream is the first treatment to receive an approved indication for use in non-segmental vitiligo with facial involvement in adults and adolescents aged 12 years and older.1,2
Currently available to private patients in the UK, Incyte is working to ensure that eligible patients will be able to access Opzelura® on the NHS.
If you have private patients who are eligible to receive Opzelura®, you can get in touch via the link below. If you are a GP and have a patient with suspected vitiligo, please refer them to a secondary care specialist or a GP with Extended Role (GPwER).
Vitiligo’s effect is not just skin deep

Often dismissed as a cosmetic, insignificant disease, in reality vitiligo patients experience a considerable psychological burden, which often impacts their mental health.3-6 Additionally, vitiligo is associated with various autoimmune comorbidities.7-9
There is currently an unmet need for treatment options for patients with vitiligo. Until Opzelura® was approved, no licensed treatments were available to aid repigmentation of the skin.3
Opzelura® – the first approved treatment for non-segmental vitiligo1,2
Opzelura®, which contains the active substance ruxolitinib, is the first approved topical JAK 1/2 inhibitor for vitiligo and has been approved for use in Europe and the UK.1,2
Opzelura® works by inhibiting JAK-STAT signalling to address the three vitiligo treatment goals:1,10
Halt depigmentation
Induce repigmentation
Prevent relapse
- Halting depigmentation – by selectively binding to JAK1 and JAK21
- Inducing repigmentation – melanocytes migrate from hair follicles and lesion edges11
- Preventing relapses – selective JAK1 binding inhibiting signal transmission by IL-1512,13
Opzelura® is the only approved treatment to aid quality repigmentation of non-segmental vitiligo.2
Significant repigmentation seen with Opzelura® in pivotal trials14
Opzelura® efficacy was examined in two randomised studies, TRuE-V1 and TRuE-V2, with results showing significant repigmentation over a long period of time.14
Facial repigmentation
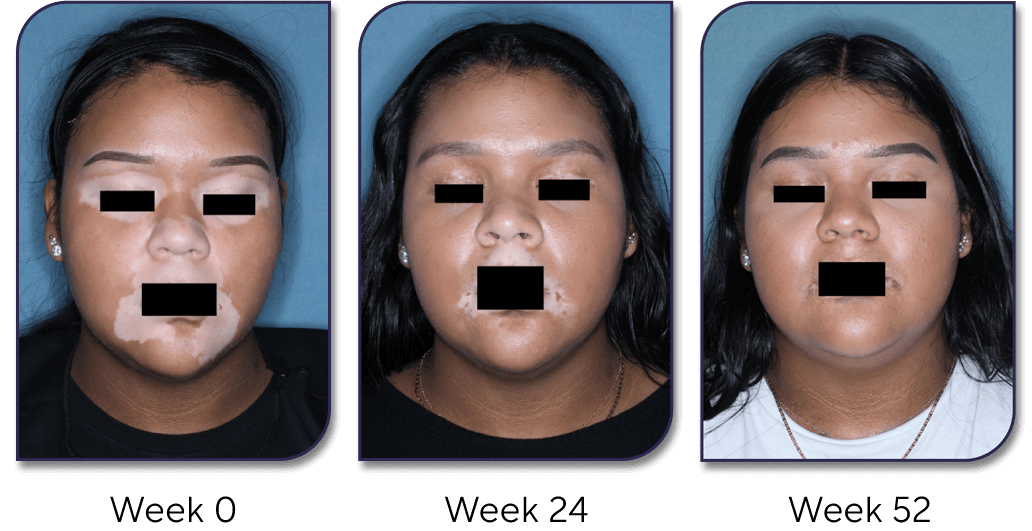
The TRuE-V1 study achieved the primary endpoint and showed significant improvement in F-VASI75 with Opzelura® vs a vehicle cream after 24 weeks [29.8% (66/221) vs 7.4% (8/109); P<0.001].14
After 52 weeks, around 30% of patients (n=173) achieved ≥90% facial repigmentation (F-VASI) with Opzelura® and one in two patients (91/173) achieved a 75% improvement in facial pigmentation.14
Body repigmentation
After 52 weeks around 50% of patients achieved ≥50% overall body repigmentation (T-VASI) with Opzelura® (92/173).14
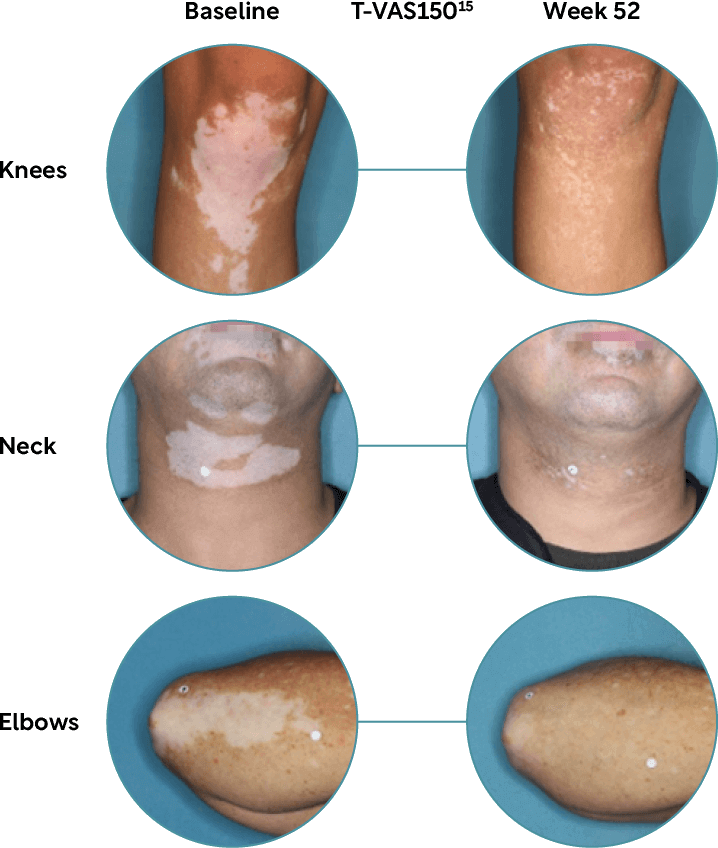
Images shown are TRuE-V1 and TRuE-V2 study participants. Individual results including level of repigmentation and time to outcome, may vary. See Prescribing Information and additional safety information.
Opzelura® cream is convenient to apply1
Opzelura® cream is applied twice a day to the depigmented skin areas up to a maximum of 10% of the body surface area (BSA).1
Opzelura® is a non-greasy formula and can be applied to sensitive areas of the skin† – making Opzelura® application convenient for your patients.1
Opzelura® was generally well tolerated in clinical trials14
In the TRuE-V1 and TRuE-V2 studies, Opzelura® showed a favourable safety profile with the most common side effect being acne at application site (5.8%).1
During the studies, no serious adverse events were considered to be related to Opzelura®.14
Your patients, vitiligo and Opzelura®
It is important to have conversations with your vitiligo patients to discuss current available vitiligo treatments, and the psychosocial impact that vitiligo may be having on their lives.
Effective vitiligo management often extends beyond medication alone. Prioritising shared decision-making and meaningful patient conversations is essential; this personalised approach ensures that treatment goals align with the vitiligo patients' preferences, lifestyles, and expectations.16
Stay informed about
Opzelura® and vitiligo
Visit our Stay informed page if you would like to receive email communications from Incyte containing information and education relevant to your clinical practice.
Incyte
dermatology
Opzelura® is manufactured by Incyte, a biopharmaceutical company on a mission to find answers for patients by following the science. Incyte is committed to discovering and developing innovative dermatology treatments to bring solutions to patients in need.
Opzelura® is contraindicated during pregnancy and breastfeeding.1
*Patient quotes are fictional.
†Opzelura® is not for ophthalmic, oral, or intravaginal use. In cases of accidental exposure to the eyes or mucous membranes, the cream should be thoroughly wiped off and/or rinsed with water.
Abbreviations
BSA, body surface area; F-VASI, Facial Vitiligo Area Scoring Index; IL-15, interleukin 15; JAK, Janus kinase; STAT, signal transducer and activator of transcription; TRuE-V, topical ruxolitinib evaluation in vitiligo study; T-VASI, Total Vitiligo Area Scoring Index.
References
- Opzelura® (ruxolitinib cream) Summary of Product Characteristics. Incyte Biosciences UK Ltd.
- Hospital Pharmacy Europe. Ruxolitinib cream gains MHRA approval for non-segmental vitiligo. Available at: https://hospitalpharmacyeurope.com/clinical-zones/dermatology/ruxolitinib-cream-gains-mhra-approval-for-non-segmental-vitiligo/ (Accessed December 2024).
- Bergqvist C, et al. J Dermatol. 2021;48(3):252–270.
- Krüger C, et al. Acta Derm Venereol. 2015;95(5):553–558.
- Bibeau K, et al. JAMA Dermatol. 2023;159(10):1124–1128.
- Phan K, et al. Pigment Int. 2022;9(2):127–130.
- Hadi A, et al. J Am Acad Dermatol. 2020;82(3):628–633.
- Gill L, et al. J Am Acad Dermatol. 2016;74(2):295–302.
- Korde S, et al. MVP J Med Sci. 2018;5(2):125–133.
- Passeron T. Dermatol Clin. 2017;35(2):163–170.
- Birlea SA, et al. Dermatol Clin. 2017;35(2):205–218.
- Chen X, et al. Free Radic Biol Med. 2019;139:80–91.
- Nolz JC, Richer MJ. Mol Immunol. 2020;117:180–188.
- Rosmarin D, et al. N Engl J Med. 2022:387(16):1445–1455.
- Incyte Biosciences International Sarl. Data on File.
- Anbar T, et al. Exp Dermatol. 2014;23(4):219–223.
UNITED KINGDOM
Adverse events should be reported.
Reporting forms and information can be found at:
www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store.
Adverse events should also be reported to Incyte by calling 00-800-0002-7423.
REPUBLIC OF IRELAND
Adverse events should be reported.
Reporting forms and information can be found at HPRA Pharmacovigilance:
www.hpra.ie. Adverse events should also be reported to Incyte by calling
1800‑456‑748.